Who Invented the Battery? From Volta to Lithium-Ion
Looking around, you will find many appliances and devices running on batteries. Battery has proven to be one of the most revolutionary inventions to facilitate this modern, electrical age. It laid the foundation of further advancement in the energy sector, offering several backup power solutions that continue to grow today.
Today, we can't imagine our lives without batteries. Be it running your household appliances to owning your cars, or things as small as charging a phone, almost everything requires a portable battery power.
But have you ever thought about who invented the battery? More importantly, how did batteries become modernized as we know them? Let us present you with all the much-needed answers!
Key Takeaways
1. Alessandro Volta invented the first true battery (the Voltaic pile) in 1800, which consisted of alternating zinc and copper discs separated by cardboard soaked in saltwater.
2. The lithium-ion battery was developed in 1985 by Akira Yoshino, who built upon earlier work by John Goodenough and Stanley Whittingham, leading to the first commercial lithium-ion battery by Sony in 1991.
Who invented the battery?
Research and studies on electricity date back to the 18th century with various scientists presenting their theories and experiments. Ever since the work published by some prominent names like Franklin Benjamin, Charles François du Fay, and Luigi Galvani, there has been a need to produce a sustainable flow of current.
Alessandro Volta, an Italian physicist, started working on this idea in the late 18th century. He developed the Voltaic Pile, a prototype of modern batteries, in 1800. The Voltaic Pile could produce a continuous flow of current, marking Volta's name in history as the "father of battery," who invented batteries.
What was the Voltaic Pile?
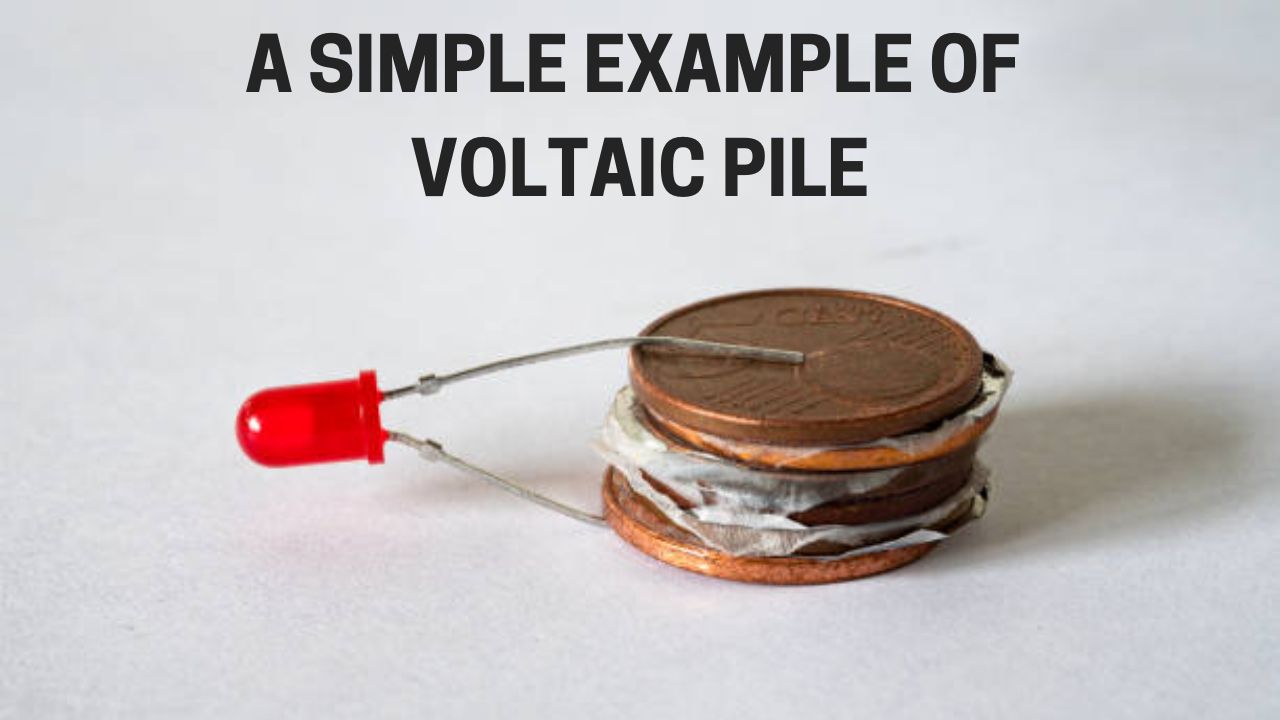
Voltaic Pile was a groundbreaking invention of its time and paved the path for modern batteries. But what was it? It was a device that used alternating Copper and Zinc discs to create a chemical reaction using paper or wet cloth as an electrolyte.
Alessandro Volta stacked these discs in an arrangement to produce equivalent power output. So, to get a higher voltage, you would have to increase the number of discs.
At its core, the Voltaic Pile was a prototype and could produce only a small amount of electricity. However, it made up for that with its continuous power output, solving a crucial problem.
When and where were batteries invented?
When were batteries invented? Some believe that batteries were being used way before Voltaic Pile. A German archaeologist, Wilhelm Konig, discovered jars sealed with an asphalt stopper, containing an iron rod in a copper cylinder. These jars were unearthed in Baghdad, Iraq, dating back to 250 BCE to 250 CE.
Although no proper evidence was found, archaeologists and many scientists believed it was a form of battery. It quickly became popular with the name, "Baghdad Battery." These jars were believed to create a small power output using the iron rod and copper cylinder arrangement. Their assumed purpose was for electroplating or electrotherapy.
The late 18th century was a curious age for electricity and magnetism. Many scientific names, including Luigi Galvani and Franklin Benjamin, emerged during these years. The Italian physician, Alessandro Volta aimed to perform practical experiments on his concept of Voltaic Pile. He invented this device at the Royal Academy of Sciences Como, Italy in 1800.
In fact, the Voltaic Pile was inspired by Luigi Galvani's work, specifically his theory of "animal electricity." Galvani experimented with a spark of electricity, touching a frog's legs to create an electric twitch. However, Alessandro Volta disagreed with this idea and started working on Voltaic Pile. He aimed to prove that electricity could be produced via chemical reactions, leading to his popular arrangement of Zinc and Copper discs.
How did the battery spread after its invention?

The 19th century saw a rise in battery usage for experimental purposes. Humphry Davy used the Voltaic Pile to separate chemical elements, leading to the discovery of potassium and sodium. In 1836, John Frederic Daniell improved the Voltaic Pile and made it more stable, making his own "The Daniel Cell."
William Robert Grove, a British scientist, used the Voltaic Pile with Platinum as its electrolyte. That led to the invention of "The Grove Cell" in 1840, serving as a building block for Telegraphy. However, Thomas Edison played a major role in innovation in the 19th century. He developed an alkaline battery, using nickel-metal hydride (Ni-MH) and nickel-cadmium (Ni-Cd).
Gaston Planté further proceeded with this idea and developed the Lead-Acid battery, marking his invention of the first rechargeable battery.
The use of these batteries increased exponentially with the rise of the Industrial Revolution in the mid-19th century. Alkaline and Lead-Acid batteries especially became popular in the 20th century with the commercialization of electronics, automobiles, and portables.
Why are batteries named the way they are?

The origin of battery comes from the French word "batterie," which means "striking in." However, it isn't the battery we know today.
The term "battery" was coined by Franklin Benjamin in 1749. Benjamin was conducting experiments with Leyden jars and static electricity. He used multiple Leyden jars to work as a unit and store electrical charges.
However, the word battery was not invented by Franklin Benjamin. The term was already popular in the military to refer to a group of artillery. Benjamin took the word battery from the military because his Leyden jars worked together to produce electricity.
Naming conventions for old and modern battery types
The first battery was called "Voltaic Pile" when it was invented. Similarly, every scientist who improved on this prototype gave it a different name. That's how we got Leclanché Cell, Daniel Cell, and Grove Cell. Gaston Planté was the earliest scientist to name his battery, "The Lead-Acid Battery" in 1859.
Naming conventions became pretty different in the 21st century. Now, batteries are no longer named after their inventors. Instead, a battery name includes several aspects, including alphanumeric codes and chemical reactions.
For example, the most common types of batteries are AA and AAA. However, they also involve a certain chemistry, as most rechargeable batteries work with NiMH (Nickel-Metal Hydride).
Who invented the lithium-ion battery?
The credit for inventing the lithium-ion battery goes to three curious minds; Stanley Whittingham, John B. Goodenough, and Akira Yoshino. In 2019, all three researchers received the Nobel Prize in chemistry for their contributions to developing lithium-ion batteries.
Here are their contributions:
1. Stanley Whittingham
Whittingham was the first chemist to start working on lithium-ion batteries. Whittingham created the first battery using lithium metal as the anode and titanium as the cathode in 1976.
Some issues were in this model. For instance, lithium is a highly reactive element that creates dendrites when charging, leading to problems like a short circuit.
2. John B. Goodenough
John B. Goodenough was a professor at the University of Oxford. He is usually credited as making the first viable lithium-ion battery.
Goodenough used cobalt oxide as the cathode in his lithium-ion battery model. Cobalt oxide showed greater stability and a better ability to store a higher density of lithium ions.
3. Akira Yoshino
Akira Yoshino, a researcher at Asahi Kasei Corporation, is credited for making lithium-ion batteries commercial.
He replaced the pure lithium metal used as the anode. Instead, he replaced it with graphite as the anode and used lithium cobalt oxide as the cathode, leaving no issues of a short circuit, or worse, an explosion.
Why did we invent the lithium battery?

Here are some key reasons behind the invention of lithium-ion batteries:
- The rising need for a portable power source surged in the late 1980s and 1990s. Previously, batteries were bulkier, leading to research on a compact battery for mobile phones, cameras, and later, laptops.
- The geopolitical situations, especially the 1970s oil crisis, also led to a demand for an alternate power source. Western countries relied heavily on oil imports, leading to a sudden surge in non-oil-reliant batteries.
- Rechargeable batteries, while reliable to some extent, had their fair share of problems. For example, a nickel-cadmium (NiCd) battery has toxic cadmium, making it environmentally hazardous.
- Lithium batteries offer unique benefits, like portability, lightweight, longer life, and rechargeability. These advantages gave them an edge over their traditional competitors.
- Renogy's smallest 12.8V 100Ah LFP Battery.
- Low-temperature cut-off for safer charging in freezing weather.
- IP65-rated & vibration-resistant.
The impact of the battery on modern technology
Without a battery, our entire society would rely on grid power. Every battery-powered, including your mobile phone or laptop, would only work while connected to a power outlet. But what if there was a blackout? Let's look at the impact of the battery on our society.
- Mobile phones & other devices: The term "mobile phone" only works because your phone is now mobile, disconnected from the power outlet. Without a battery, it would be impossible to make mobile phones. The same goes for tablets, smartphones, and laptops.
- Health industry: Many life-saving medical devices, including pacemakers and defibrillators, use batteries. Batteries extend the lifespan of these implants, enabling them to stimulate biological functions in the body. Similarly, many other devices, like heart rate and glucose monitors, have batteries to monitor bodily performance and provide insights remotely.
- Energy sector: The energy sector thrives on batteries, eliminating energy waste. Batteries store energy to use when it is needed. Additionally, it reduces grid power reliance by integrating with power backup options, like solar panels.
- Battery-powered vehicles: Originally, cars used steam engines and combustion mechanisms to work. However, these methods had issues like a shorter driving range and environmental problems. With the integration of batteries, modern vehicles have become more efficient.
Conclusion
So, next time someone asks you who invented the battery, you know the answer! Many scientists, chemists, and researchers have played key roles in the invention of batteries. Alessandro Volta started this journey of modernizing the backup power, soon joined by Franklin Benjamin, Humphry Davy, John Frederic Daniell, William Robert Grove, and Gaston Planté, each further advancing the idea.
Later on, lithium-ion batteries came into the picture and changed the entire battery landscape, thanks to Stanley Whittingham, John B. Goodenough, and Akira Yoshino.
But that's not the end. The future also holds many advancements for the battery. Currently, the industry focuses on battery recycling, grid-scale storage, and solid-state batteries. The future promises sustainability, eco-friendly manufacturing, and technological integration.