What Element is Used in Batteries? Explore Chemistry of Battery
Batteries are the backbone of all electronic devices. They have become an essential part of our daily lives as they are frequently used in a wide range of devices, from toys to mobile phones, cameras to laptops, and electric vehicles to medical gadgets and smartwatches.
Simply put, we can't even think of our lives without batteries. A range of batteries are available in today's market. Each comes with a different chemistry, different elements, different warranty, and different life cycle.
Knowing battery composition is important because it tells you how efficient, safe, and reliable a battery will be. The price of each battery type varies, depending on factors like capacity, support for charge cycles, energy density, lifespan, and self-discharge ability.
In this article, we will discuss what element is used in batteries of different types and how they are different from each other. It also explains why battery elements matter.
What element is used in batteries?
Different elements are used in different batteries, considering the current market demands and the efficiency of each element. The most commonly used metal-based elements in various batteries include Lithium (Li), Cobalt (Co), Nickel (Ni), Cadmium (Cd), Lead (Pb), Sodium (Na), Zinc (Zn), Manganese (Mn), and more.
The availability and cost of each element is different, which is why the manufacturing cost of batteries developed with different materials will also be different.
Li-based batteries often need a protection circuit. When lithium is used in batteries in the intended manner, it is not dangerous to human health. The leading plus point of using nickel in batteries is that it helps them deliver higher storage capacity and greater energy density at a fair cost. In comparison, lead-based batteries offer high surge currents and help manufacturers maintain low costs.
What are batteries made of?
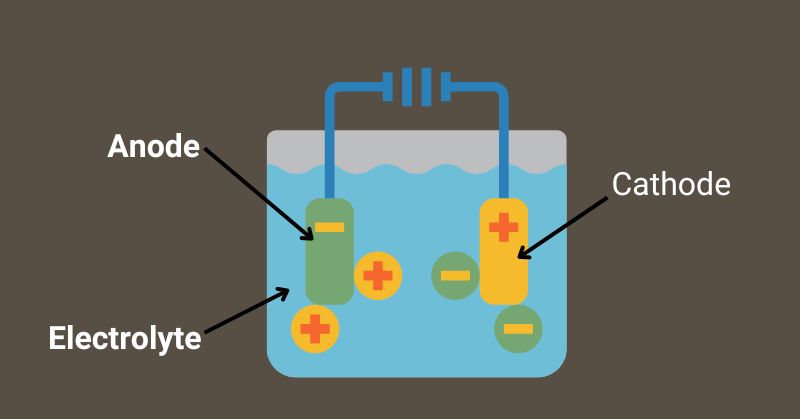
Batteries are made up of three main components: anode, cathode, and electrolyte. Each component has to perform unique functions. Both anode and cathode are also called electrodes.
The cathode is a positive, and the anode is a negative electrode. Reduction and oxidation occur on the cathode and anode, respectively.
Let's look at what cathode, anode, and electrolyte are composed of in modern batteries.
1. Lithium-ion batteries
Lithium is used as a primary active material for manufacturing both the cathode and anode of a lithium-ion battery. To produce electricity, lithium ions flow through the electrolyte from the anode to the cathode.

Usually, the anode in lithium-ion batteries is made up of graphite, whereas the cathode is made of lithium iron phosphate, lithium cobalt oxide, or other similar compounds. Lithium salt is mostly used as an electrolyte. These batteries are known for their extended lifespans and unmatched energy density. They are mostly used in electric vehicles, smartphones, and other similar devices.
2. Lead-acid batteries
In a lead-acid battery, lead dioxide makes up the cathode, whereas the anode is made up of a sponge. Both electrolytes are dipped into the sulfuric acid solution - the electrolyte. These batteries belong to the family of rechargeable batteries, which is why they are also called secondary batteries.

The chemical reactions that occur within the battery are reversible. The most common applications of these batteries include vehicles and Uninterruptible Power Supplies (UPS). They are also a great choice for backup power systems.
3. Nickel-cadmium batteries
A nickel-cadmium battery is also known as a NiCd battery. In this battery, the cathode is made of nickel oxyhydroxide, and the anode is built with cadmium. The alkaline solution with potassium hydroxide serves as an electrolyte.

The cadmium facilitates the flow of electric current when it undergoes oxidation at the anode. The cathode accepts electrons, which is why nickel oxide is reduced. These batteries are highly robust and reliable and are mostly used in portable power tools and cordless phones.
Comparing the materials of rechargeable and non-rechargeable batteries
The table below shows the comparison between the materials of two rechargeable and non-rechargeable batteries.
| Rechargeable batteries | Non-rechargeable batteries | |||
| Materials | Li-ion | NiMH | Alkaline | Zinc-carbon |
| Cathode | Lithium | Nickel hydroxide | Manganese dioxide | Manganese dioxide |
| Anode | Graphite | Hydrogen | Zinc powder | Zinc |
| Electrolyte | Lithium salt | Potassium hydroxide | Potassium hydroxide | Zinc chloride |
Why do battery elements and materials matter?
Different batteries are made up of different primary and secondary elements, such as lithium, nickel, lead, cadmium, manganese, and more. Each element possesses different properties that affect the overall composition of the battery.
For example, lithium is the least dense solid material and the lightest metal. It is used as the anode material in lithium-based batteries. Due to its high electrochemical potential, lithium is considered a key element in rechargeable Li-ion batteries that come with high energy density.
The use of lead in batteries makes them reliable. Lead submerges into a sulfuric acid solution to produce a controlled chemical reaction. As a result, electric current is produced. Lead-based batteries are eco-friendly and convenient to use. Similarly, nickel can deliver high energy density and large storage capacity due to its higher specific energy. This cheap metal forms the cathode of different rechargeable batteries, including Li-ion batteries.
Simply put, the battery elements and materials matter the most, as they are the ones that actually tell the energy density, energy capacity, self-discharge ability, lifespan, safety, cost, long autonomy, and other similar features of a battery. You can explore tremendous battery collection at Renogy.
Advancements in battery materials and technologies
We have seen continuous advancements in battery materials and technology. For example, many battery manufacturers have stopped developing cadmium-based batteries (such as nickel-cadmium), as they are hazardous to the environment and are toxic.
Similarly, lead-acid batteries offer a relatively shorter life cycle (mostly less than 500 charge cycles) compared to other modern batteries.
In comparison to nickel-cadmium and lead-acid batteries, lithium-ion batteries are far better due to their low self-discharge ability and higher energy density. Along with these benefits, some drawbacks are associated with lithium-ion batteries, such as bursting risks, higher costs, and sensitivity to high temperatures.
Researchers are continuously working on improving battery technology and materials. The seven modern technologies that have the ability to replace lithium-ion batteries in the future include lithium-sulfur batteries, graphene batteries, solid-state batteries, sodium-ion batteries, zinc-based batteries, cobalt-free batteries, and iron-air batteries. Let's look at them one by one.
1. Lithium-sulfur batteries
These batteries offer higher efficiency and high energy storage capacity for electric vehicles. In addition, sulfur is abundant and cost-effective; therefore, it helps manufacturers make budget-friendly energy storage solutions compared to lithium-ion batteries. The manufacturing of both lithium-ion and lithium-sulfur batteries follows a similar process, so manufacturers can use the same facility.
2. Graphene batteries
Compared to lithium-ion batteries, graphene batteries are more conductive. These batteries support faster charging and offer extended life cycles than their counterparts. Moreover, the structure of graphene is sturdy, which is why it makes the battery more reliable than its lithium-ion alternative. Therefore, graphene batteries have lower risks of fires and explosions.
3. Solid-state batteries
Solid-state batteries can store more energy in the same size than lithium-ion batteries. In addition, they are more efficient and lightweight. Electric vehicles can be more compact and lighter with these batteries installed. Furthermore, solid-state batteries are charged faster than Li-ion batteries. They are believed to be safer and longer-lasting.
4. Sodium-ion batteries
A sodium-ion battery is very safe and affordable compared to its counterpart, Li-ion battery. Though it offers low energy density, it performs very well at low-temperature levels when compared to a lithium-ion battery. Researchers are currently working on enhancing the charging time and efficiency of sodium-ion batteries.
5. Zinc-based batteries
The materials used in the manufacturing of zinc-based batteries are readily accessible, cost-effective, and non-toxic. Additionally, these batteries can store an abundance of power. Research scientists are still working on fixing some technical issues associated with this modern battery technology, such as the potential of short-circuiting. More research is required before they become widely accepted.
6. Cobalt-free batteries
The mining of Cobalt is associated with human rights abuses. Plus, this is a highly costly material. Therefore, the leading benefit of these batteries is that they do not use Cobalt. According to the US Department of Energy, they are hoping to stop the use of Cobalt in lithium-based batteries by 2030.
7. Iron-air batteries
Both iron and air are present in abundance in the world. Therefore, iron-air batteries are incredibly affordable. According to Popular Mechanics, an iron-air battery can last up to 17 times longer and is up to 10 times more affordable than a Li-ion battery. Slow recharge time and larger size are the two limitations associated with these batteries.
If you are looking for a battery that comes with a self-heating feature and built-in Bluetooth to ensure seamless connectivity with mobile devices, look no further than a 12V 200Ah Pro Smart Lithium Iron Phosphate battery at Renogy.
Conclusion
People who are in search of how to choose the right battery often ask the question, “What element is used in batteries?” Well, a battery is made of different components, such as the cathode, the anode, and a solution (electrolyte). The cathode and anode are known as electrodes, and they are formed with different elements, including but not limited to lithium, nickel, cobalt, manganese, lead, cadmium, and sulfur.
The composition of a battery varies depending on the elements and materials used during the manufacturing process. Both materials and elements of batteries tell how long they can last, how fast they can charge, how much energy they can store, how safe they are, how quickly they can discharge, and how they perform in extremely high or low temperatures.
Scientists have developed a number of new battery technologies (as discussed above) and researchers worldwide are working on further improving the newly introduced batteries to make them widely acceptable.
FAQs
What is the most common element used in batteries?
Lithium (Li) is the most commonly used element in both rechargeable (in EVs, laptops, and mobile phones) and non-rechargeable (in clocks, toys, and heart pacemakers) batteries.
Can battery materials be recycled?
Not all, but yes, the materials used in most battery types can be recycled. Nowadays, advanced recycling processes can recover around 25-96% of lithium-ion battery materials. The recycling processes are complex and need extended safety.
Which battery type is most environmentally friendly?
According to many research studies, lithium-sulfur batteries are the most environmentally friendly batteries for electric vehicles. They significantly reduce the emission of greenhouse gasses and thus save the air from pollution (unlike other fossil fuels).