How Do Lithium Ion Batteries Work? A Step-by-Step Explanation
Lithium-ion batteries have become an integral part of our daily lives, powering everything from smartphones and laptops to electric vehicles and home energy storage systems. But how exactly do these batteries work?
In this article, we'll delve into how do lithium-ion batteries work, exploring their key components, charging and discharging processes, and the factors that influence their performance. By understanding how these batteries operate, we can better appreciate their role in shaping our modern world.
What are the components of a lithium-ion battery?
Lithium-ion batteries are comprised of several key components that work together to store and release electrical energy. These components include:
- Cathode: The positive electrode of the battery, typically made of materials like lithium cobalt oxide (LCO), lithium nickel manganese cobalt oxide (NMC), or lithium iron phosphate (LFP).
- Anode: The negative electrode of the battery, often made of graphite or silicon.
- Electrolyte: A liquid or gel-like substance that allows ions to move between the cathode and anode during charging and discharging.
- Separator: A porous membrane that separates the cathode and anode, preventing direct contact while allowing ions to pass through.
- Current Collectors: Conductive materials (usually copper for the anode and aluminum for the cathode) that collect electrical current from the electrodes.
Each of these components plays a crucial role in the operation of a lithium-ion battery, and their specific materials and design can significantly influence the battery's performance, capacity, and lifespan.
Note: Still unsure what element goes into your everyday batteries? Dive deeper and discover why lithium reigns supreme in powering our devices! Learn more about lithium-ion battery technology here: What Element is Used in Batteries?
How lithium-ion batteries work?
At the core of a lithium-ion battery, positively charged lithium ions move through an electrolyte from the anode (negative side) to the cathode (positive side), and back again, depending on whether the battery is charging or discharging. This ion movement triggers the release of free electrons in the anode, generating electrical energy. The free electrons flow from the positive current collector, powering the connected device, and then return to the negative current collector. Meanwhile, a separator within the battery ensures that electrons don't flow freely between the anode and cathode, which prevents short circuits and ensures safe operation.
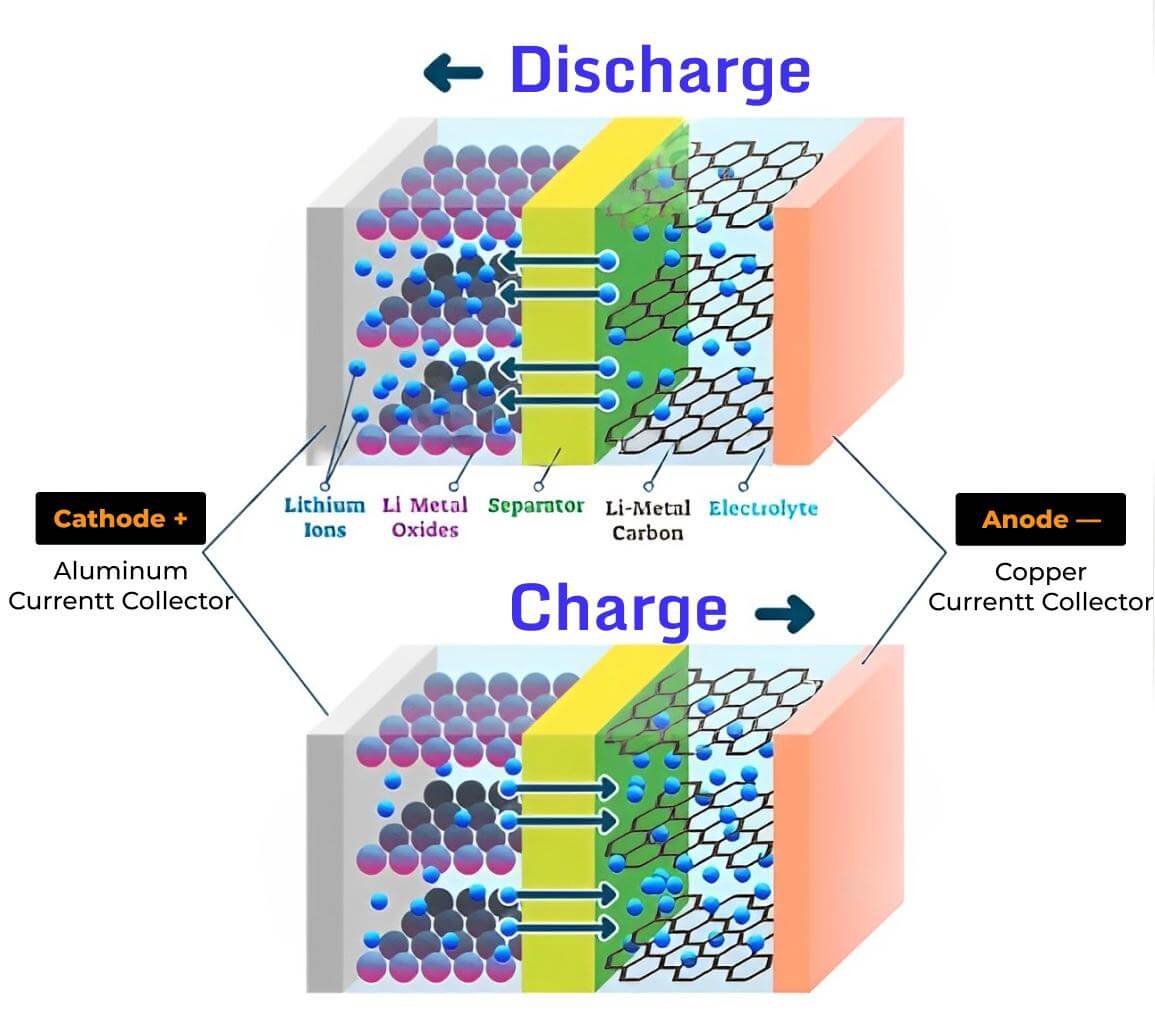
How a lithium-ion battery charges and discharges
When a lithium-ion battery is charging, lithium ions move from the cathode (positive electrode) to the anode (negative electrode) through the electrolyte. The anode, usually made of graphite, acts as a host for these lithium ions, which get stored in its layered structure. At the same time, electrons are forced to move through an external circuit from the positive current collector to the negative one, driven by the applied voltage. This process stores electrical energy in the battery.
During discharge, the process reverses. The stored lithium ions move back from the anode to the cathode, releasing the stored energy. As these ions migrate, the free electrons that were once pushed into the external circuit now return, flowing through the device the battery powers. This flow of electrons is what provides electricity to power devices like smartphones, laptops, and electric vehicles.
The charging and discharging process continues until all the lithium ions are moved or until the battery reaches its maximum capacity, at which point it must be recharged to continue working efficiently.
How are the lithium ions stored
In a lithium-ion battery, the lithium ions are primarily stored in the anode and cathode. These components are made of different materials to hold and release lithium ions as needed. When the battery is in a charged state, lithium ions are embedded in the anode material, often graphite. The anode's layered structure allows for a high concentration of lithium ions to be stored between its layers.
On the other hand, the cathode, typically composed of a metal oxide (such as lithium cobalt oxide or lithium iron phosphate), stores lithium ions when the battery is in a discharged state. The ions shuttle back and forth between these two components during charging and discharging, which enables the battery to store and release energy efficiently.
The choice of materials in both the anode and cathode is critical because it determines the battery's capacity, lifespan, and performance. This storage mechanism is what makes lithium-ion batteries more efficient than other battery types, as they can pack a lot of energy in a small space and are highly rechargeable.
Advantage of using a lithium-ion battery
Lithium-ion batteries have become the power source of choice for a wide range of modern technologies, from portable electronics to electric vehicles and renewable energy systems. Here are the key advantages that set lithium-ion batteries apart:
Higher Energy Density
Lithium-ion batteries offer a much higher energy density than traditional batteries like lead-acid. This means they can store more energy in a smaller, more compact design. For devices like smartphones, laptops, and even electric cars, this higher energy density allows for longer usage times and improved overall efficiency without taking up too much space.
Lightweight
A major advantage of lithium-ion batteries is their lightweight structure. They weigh significantly less than lead-acid batteries, making them ideal for portable applications like smartphones, power tools, and drones. In electric vehicles, the lighter weight helps enhance performance, increase mileage, and improve handling.
Longer Battery Life
Lithium-ion batteries have an extended charge cycle life, meaning they can be charged and discharged many more times before losing capacity. This reduces the need for frequent battery replacements, offering long-term savings. With proper usage, a lithium-ion battery can last several years without significant performance drops.
Fast Charging
One of the biggest benefits of lithium-ion technology is its fast charging capability. Devices and electric vehicles can recharge quickly, which is essential for minimizing downtime. Whether you're on the go with your smartphone or charging an electric vehicle, faster charging saves time and adds convenience.
Low Maintenance
Lithium-ion batteries are virtually maintenance-free. Unlike lead-acid batteries, they don't require regular water level checks, cleaning, or specific charging cycles to prevent damage. Plus, they are immune to sulfation, which can cause lead-acid batteries to degrade if not properly maintained.
Future Development of lithium battery
Today's lithium-ion rechargeables offer significant advantages over their predecessors, but the journey toward perfect energy storage is far from complete. Despite their impressive performance, challenges such as thermal runaway remain a concern. As the urgency of climate change intensifies, the demand for batteries that are cheaper, safer, more energy-dense, and environmentally friendly becomes increasingly critical. Researchers are racing to develop batteries that charge faster, pack more energy into smaller spaces, and minimize their environmental footprint. Exciting advancements are on the horizon, including ultra-fast-charging graphene batteries, nanomaterial-based batteries, and even biologically inspired batteries utilizing genetically engineered viruses and vitamins. These innovative technologies could soon power our devices, marking a new era in energy storage.
- The 2-in-1 12V 200Ah LiFePO4 battery with self-heating & BT
- Flame-retardant casing stops vertical burning from spreading in 10sf
- Waterproof, dust-proof (IP67), and corrosion-resistant (ISO 9227)
Conclusion
Lithium-ion batteries power our modern world, from smartphones to electric vehicles. These innovative energy storage devices rely on the movement of lithium ions between positive and negative electrodes to generate electricity. Their high energy density, long lifespan, and quick charging capabilities make them ideal for various applications. Companies like Renogy have embraced this technology, offering reliable lithium-ion battery solutions for solar energy systems and off-grid living. As research continues, lithium-ion batteries are becoming more efficient, safer, and sustainable. Understanding how these batteries function is crucial as we move towards a greener future. By grasping the principles behind lithium-ion technology, consumers can make informed decisions about their electronic devices and energy storage needs.
FAQs about lithium battery
What are the disadvantages of lithium-ion batteries?
Lithium-ion batteries, while highly efficient, have some drawbacks. One major concern is their potential for thermal runaway, which can lead to fires or explosions under certain conditions. Additionally, they have a limited lifespan and may degrade over time, especially when subjected to extreme temperatures or frequent charging cycles.
What is the largest problem with lithium-ion batteries?
The largest problem with lithium-ion batteries is their potential for thermal runaway. This occurs when the battery's internal temperature rises uncontrollably, leading to a chain reaction that can result in fires or explosions. While advancements have been made to mitigate this risk, it remains a significant challenge in the industry.
Can lithium batteries really be recycled?
Yes, lithium batteries can be recycled, but it's a complex process. The valuable materials within the batteries, such as lithium, cobalt, and nickel, can be extracted and reused to manufacture new batteries. However, the recycling process requires specialized equipment and techniques to ensure safety and environmental responsibility. Recycling lithium batteries is essential for reducing waste and conserving natural resources.